Abstract
Introduction Hyperviscosity syndrome (HVS) is a rare and life-threatening complication of multiple myeloma (MM). HVS was a criterion of MM treatment in the previous international recommendation, but it was withdrawn in the IMWG 2014 guidelines. Moreover, studies about the prognosis of HVS in modern-era therapy are missing. Our objective is to describe MM patients with HVS and to evaluate its prognosis impact.
Methods Inclusion criteria for the case were patients aged ≥18 years newly diagnosed with MM with HVS in Saint Louis Hospital, Paris, France, between 2011 and 2021. HVS diagnosis was based on presence of neurological signs and/or evaluation by ophthalmologic examination for symptoms or high spike. Two controls were selected for each case in the same period with the best fit set on age, sex, year of diagnosis, and front-line treatment. The primary outcome was overall survival.
Results Thirty-nine MM patients with HVS (7 severe forms and 32 ophthalmologic confirmation) were included with a median age of 59 years (IQR 51-68). The main isotype of immunoglobulin was IgG (69%, median 61 g/L), followed by IgA (28%, median 41 g/L). Translocation t(11;14), presented in 37% of the cohort (13 IgG and 1 IgA), was associated with a higher spike level than other patients (for IgG: 71 versus 59 g/L, p=0.0001). HVS clinical presentation was heterogenous: 23% asymptomatic (median spike at 60 and 44 g/L for IgG and IgA), 59% mild (epistaxis, headache, blurry vision) and 18% severe with neurological signs, respectively. Fifteen (38%) patients required ICU admission: severe HVS for 6, association with other complications for 6 and only for therapeutic plasma exchange (TPE) realization for 3. No major ischemic or bleeding was observed at diagnosis. For HVS management, in addition to myeloma therapy, TPE was performed for 35 patients (92%), which was well tolerated, and 3 patients received only steroids. One month after HVS diagnosis and care, no rebound effect of monoclonal spike was observed and 33 (84%) patients had complete clinical recovery. Three patients (8%) died, and three (16%) had an ophthalmic sequela of HVS.
All patients had at least one CRAB criterion except one who had two focal lesions on MRI. Most of the patients received bortezomib and high-dose steroids (95%) associated with an immunomodulatory drug (43%) or alkylating agents (42%). Two patients (5%) received Daratumumab. For younger patients (< 65 years), 21 patients (84%) received autologous stem cell transplantation upfront. After the exclusion of one patient with an early loss in follow-up (< 3 months), the global response rate (≥PR) was 92%. The median time to the subsequent treatment was 2.1 years (95%IC 1.4-3.4). At subsequent relapse, 6 patients (3 IgG, 2 IgA and 1 IgM) had HVS recurrence.
With a median follow-up of 5.2 years, the median OS for HVS patients was 3.6 years (95%CI 2.7-not reached). Among patients older than 65 years, the early mortality rate (< 3 months) was 21%. Presence of > 5% of blood plasma cell (HR 6.06, p=<.01), high-risk cytogenetics (HR 3, p=0.03), R-ISS3 score (HR 3.3, p=0.04), and LDH above normal level (HR 3.6, p=0.01) shortened the OS.
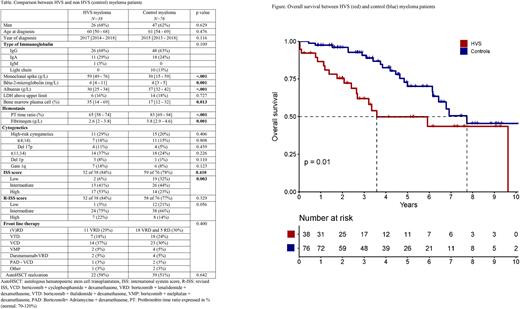
Compared to controls, HVS MM patients had a higher spike level at diagnosis, higher ISS3 rate, higher bone marrow plasma cells and lower fibrinogen levels (Table). HVS myeloma had dismal OS compared to controls (median: 3.6 vs. 7.7 years, p=0.01, Figure). In a multivariate analysis of pooled HVS and control patients, HVS (HR 2.7, 95%CI 1.4-5.4, p=0.004) and high-risk cytogenetics (HR 2.8, 95%CI 1.4-5.7, p=0.005) were two independent adverse factors for OS. HVS remained an independent factor for OS for myeloma with the addition of the ISS score or R-ISS score in the model.
Conclusion HVS at myeloma diagnosis is a rare but urgent complication associated with high lethality in older patients. HVS was associated with high tumor burden and dismal survival, independent of principal prognosis scores in modern treatment era.
Disclosures
Brignier:OCTAPHARMA: Research Funding; SANOFI,: Research Funding; GILEAD-KITE: Research Funding; AMGEN: Research Funding; THERAKOS: Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; JANSSEN: Honoraria, Membership on an entity's Board of Directors or advisory committees. Arnulf:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal